Give the Number of Valence Electrons for Xecl4
For output press the Submit or Solve button. When we put 4 F atoms around 1 Xe atom each Flourine atom is going to want to share one of Xenons.
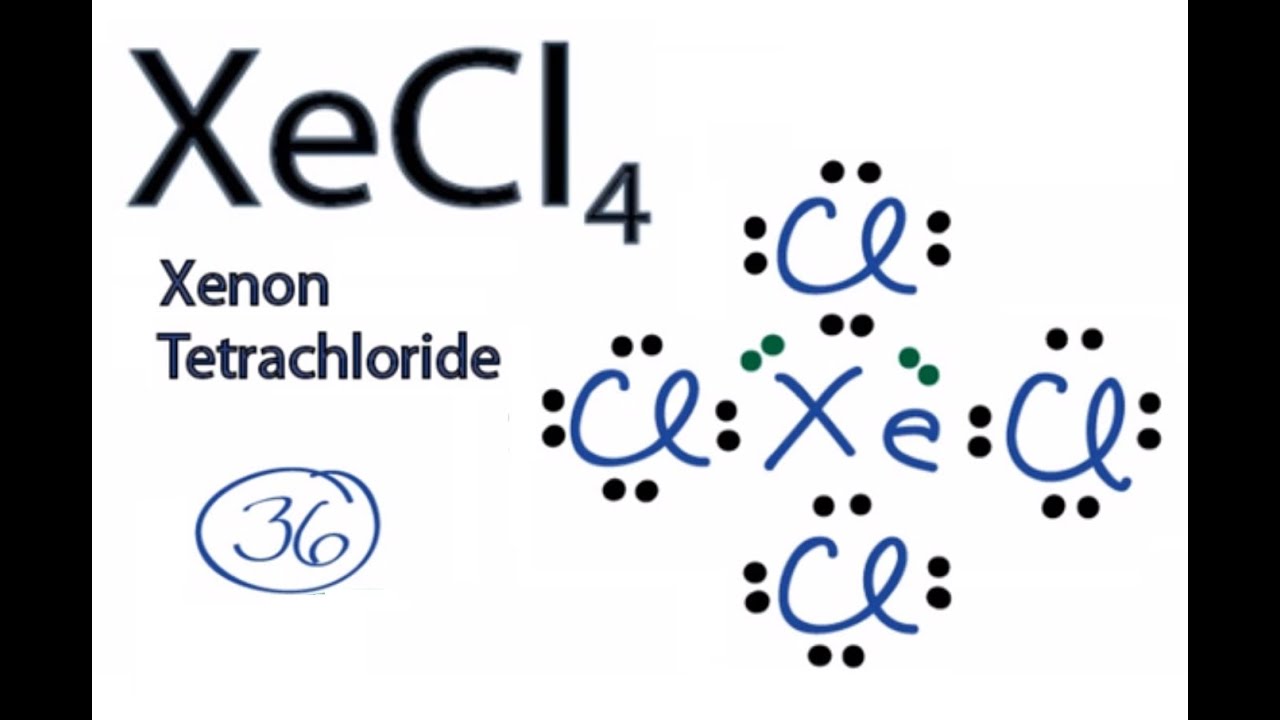
Xecl4 Lewis Structure How To Draw The Lewis Structure For Xecl4 Xenon Tetrachloride Youtube
5 electrons for N 6 times 3 for O and 1 for the extra negative charge.

. Therefore 74 shall give us 28. Choose the compound below that should have the lowest melting point according to the ionic bonding model. So thats 2 bonds right there.
Chlorine atoms in a demands electrons. Total outermost valence shell electrons available for XeCl4 Lewis structure dot structure 847 36 valence electrons in XeCl4. D fill in the remainder of the information requested in the table.
Basically count up the lines and the pairs of dots. Atoms are most stable if they have a filled valence shell. XeF4 Valence electrons.
Follow the below steps to get output of Valence Electron Calculator. A 0 lone pairs linear. A molecule containing a central atom with sp3d2 hybridization has an _____ electron geometry.
Xenon tetrachlorideXeCl4 has the composition of one xenon and three chlorine atoms. A triple covalent bond contains _____ of electrons. And hybridization for XeCl4.
7x2 14 6 20 electrons in total Each Fluorine makes a single bond with the oxygen atom. Give the number of valence electrons for SI4. Then the total outermost valence shell electrons can be calculated as follows.
Three Cl atoms are bonded to three single electrons and one electron is added to the remaining single electron to give the negative charge on the ion. Youll want to calculate the formal charges on each atom to make sure you have the best Lewis structure for XeCl 4. Egtetrahedral mgtrigonal pyramidal sp3.
Thats it Now your window will display the Final Output of your Input. Chemistry questions and answers. Steps to use Valence Electron Calculator-.
Determine the total number of valence electrons in bromine pentafluoride BrF5. Identify the number of electron groups around a molecule with a trigonal bipyramidal shape. 1 For each of the following.
The number of valence electrons of an atom can be obtained from the periodic table because it is equal to the group number of the atom. Identify the molecular geometry. Valence electrons of Xenon 8.
Hence there are 36 valence electrons in the compound XeF4. What are the approximate bond angles in XeCl4. The number of valence electrons in neon is 8 and its valence electronic configuration is 2 s2 2 p6.
In this molecule we have one atom of Xenon and four atoms of Fluorine. Choose the best Lewis structure for NH4. Characteristics of Valence Electron.
XeCl4 CH4 SF4 C2Cl2. The central Xe atom has 4 bond pairs of electrons and two lone pairs of electrons. Xenon Xe can have more than 8 valence electrons in your Lewis structure.
A The atomic number of neon is 10 and its electronic configuration is 1 s2 2 s2 2 p6. Identify the molecular geometry of XeCl4. Give the chemical symbol for the element with the ground-state electron configuration Ne3s2 3p3.
Choose the bond below that is. Give the electron geometry molecular geometry and hybridization for NH3. C calculate the formal charge for each atom in each molecule and add it to the Lewis Structure.
Up to 24 cash back add valence electrons to the four chlorine atoms and the final step is to combine the step1 and step2 to get the XeCl4 Lewis Structure. The valence electrons of O is 6 and F is 7. B draw the Lewis Structure include all resonance structures.
It is said to occupy orbitals in an atom. Xe 8 valence e-Cl 7 valence e-x 4. In the input field enter the required values or functions.
The XeCl4 molecule has one central xenon and four chlorine atoms. Give the electron geometry eg molecular geometry mg and hybridization for SCl2. Electrons are involved in the chemical bonding and reactions of the atom.
The Lewis structure for XeCl 4 requires you to place more than 8 valence electrons on Xe. Step 1 of 3. XeCl4 CBr4 SF4 C2H2 a.
Hint draw the Lewis structure for XeCl4. Valence electrons of Fluorine 74 as there are four Fluorine atoms we will multiply it by 4. The total valence electrons of XeF4 come to be 828 which is 36.
6Se 12O 18 valence electrons. The XeCl4 Lewis structure is a diagram that illustrates the number of valence electrons and bond electron pairs in the XeCl4 molecule. Remember the geometry of a molecule has to do with how many electron pairs AND lone pairs there are around the CENTRAL atom.
XeCl 4 has a total of 36 valence electrons. A eg tetrahedral mg tetrahedral sp3 B eg trigonal. N single bonded to 4 H 8 total electrons.
Identify the number of electron groups around a molecule with sp3 hybridization. An XeCl4 molecule is _____. A find the number of valence electrons.
Carbon is surrounded by 4 electron groups. We will calculate the valence electrons of both these atoms to determine the total number of valence electrons of XeF4. Thus the total number of valence electrons in Selenium Dioxide SeO 2 is given by.
Give the number of valence electrons for CH2Cl2. Notice that Xe has 8 electrons in its valence shell and F has only 7. Xenon shows the least electronegative property.
More Online Free Calculator. What is the molecular geometry of xenon tetrachloride. Oxygens electronic configuration is 1s 2 2s 2 2p 4.
Therefore two Oxygen atoms contribute 6 x 2 12 valence electrons. The geometry of the XeCl4 molecule can then be predicted using the Valence. Give the number of valence electrons for CH2I2.
A SO 2 OS O-1 1 OS O-1 1 O S O 18 e-b SO 3. Chlorine and xenon have seven and eight valence electrons respectively. Give the number of lone pairs around the central atom and the molecular geometry of SCl2.
Being in group 6 of the periodic table Oxygen has six valence electrons and has a valency of -2. So the total number of valence electrons is 24.

How To Draw Xecl4 Lewis Structure Science Education And Tutorials

How To Draw Xecl4 Lewis Structure Science Education And Tutorials

Solved Give The Number Of Valence Electrons For Xecl4 38 O Chegg Com

How To Draw Xef4 Lewis Structure Science Education And Tutorials
Comments
Post a Comment